Healthcare Provider
Testing
GENETWORx COVID-19 Diagnostic and Antibody Tests for Healthcare Providers
As a CLIA-certified and CAP-accredited laboratory, GENETWORx is your full-service provider for COVID-19 Diagnostic and Antibody Tests. Our diagnostic tests detect the presence of COVID-19 at a level of sensitivity that surpasses other laboratories. We will have testing supplies in your hands within 24 hours, and results in less than 72 hours.

Order Testing for
Healthcare Providers Today
COVID-19 Diagnostic Tests
Physicians, hospitals, and any other United States healthcare provider can order the GENETWORx 2019 Novel Coronavirus (COVID-19) test to provide patients with quick, accurate results.
The GENETWORx 2019 Novel Coronavirus (COVID-19) nasopharyngeal test provides 99% specificity and sensitivity for detection of the presence of SAR-CoV-2, the virus responsible for COVID-19. Our tests follow the Centers for Disease Control approved assay kits.
GENETWORx can mail COVID-19 diagnostic self-collected nasal swab test kits to the homes of your employees or patients. The GENETWORx diagnostic COVID-19 Nasal Swab Test Kit received FDA emergency use authorization and is convenient, accurate and safe. With your order, employees or patients receive a test kit to their preferred address with the supplies and instructions needed to collect and return a test, as well as the FedEx return mailer. Results are shared electronically with both employer and employee through an easy-to-use app, Aura, within 48 hours of lab receipt. Click here to learn more about this test.
For more information on our testing capabilities, please contact GENETWORx by calling 866-932-0109 or completing the Order COVID-19 Tests form.

My facility has been using GENETWORx for several months now and we are beyond satisfied. The lab testing and reporting is exceptional. The customer service is phenomenal. We have not had any issues and are very happy to have found a lab to partner with in order to provide the best patient care possible. Our results are always available much more quickly than other labs we have looked into and there is always someone available to assist with any questions we have.”
– Zoe Jenkins, MA, MT (AMT) Laboratory Services Director and Facility Joint Commission Coordinator.

GENETWORx is currently accepting COVID-19 diagnostic and antibody test orders and samples from medical facilities, healthcare providers, governments, universities, and colleges, nursing homes and assisted living facilities, and all employers throughout the United States. Learn how to order tests below.
COVID-19 Total Antibody (IgM & IgG) Tests
The GENETWORx COVID-19 Total Antibody (IgM & IgG) assay is a blood test that is performed to determine if a patient has been exposed/infected with the COVID-19 virus.
The COVID-19 Antibody Test checks to see if a patient’s immune system has responded to the infection. It is not for diagnosing an active COVID-19 infection. The GENETWORx Assay test is most specific and sensitive when it is performed at least 14 days after infection from COVID-19. However, if someone does not have symptoms but suspects they may have been infected, this test can be conducted at any point.
The presence of antibodies to SARS-CoV-2 indicates that the patient, whether the person experienced symptoms or not, had an immune response to the virus. GENETWORx’s Total Antibody tests detect both IgG and IgM in the blood to provide a clearer disease-state picture. When it comes to early detection of an immune response, our assays are much more sensitive – 99.8% specific and 100% sensitive to be exact.
Ready to order Antibody tests? Call 866-932-0109 and we’ll have your testing kit to you within 24 hours.
Order Testing for
Healthcare Providers Today


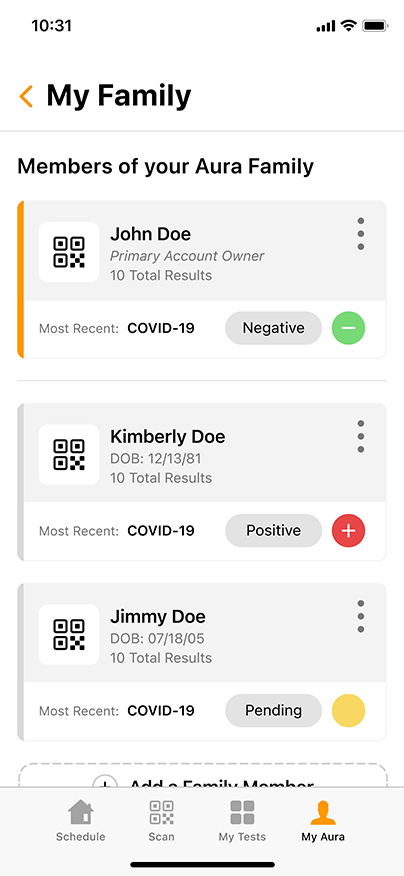
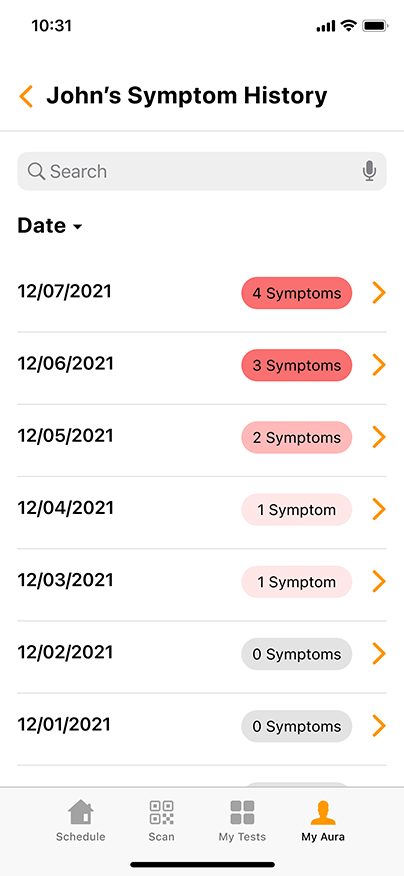
Aura: The COVID-19 Reporting and Security Software
Aura, the proprietary COVID-19 reporting and security software from GENETWORx, tracks sequential testing results and provides dashboards, accurate reporting, and important health data reflecting the COVID-19 status of your business.
Aura can easily integrate with your business’ security system, enabling you to screen for untested and coronavirus-positive persons at entry points to your workplace. Using standard QR-readers, Aura can cross reference individuals entering the workplace with their most recent COVID-19 test results, creating a discrete and effective way to suppress the presence of the contagion.
Aura is HIPAA compliant and can be customized to meet the needs of your specific business. GENETWORx can conduct independent assessments of your workplace and provide tailored security solutions to greatly mitigate the risks associated with COVID-19.
To learn more about Aura, click here.
How to Obtain On-site COVID-19 Diagnostic Test Kits for Your Organization

Contact GENETWORx
Contact GENETWORx. Within 72 hours, an account will be created and you will receive access to the GENETWORx portal for future test results.
Receive Test Kits
Within 24 hours the healthcare provider (HCP)/practice designated will receive a nasopharyngeal diagnostic test kits, or antibody testing supplies (if needed). The nasopharyngeal diagnostic test kit will include a nasopharyngeal swab, and the universal transport media in a vial to ensure RNA and DNA survival. Kits include a biohazard bag and postage paid, pre-addressed FedEx return pack.

Send Test Kits Back to GENETWORx
Once the test is administered (by the HCP for the nasopharyngeal test), the HCP or individual will put the vial and the test order with the ordering-physician’s signature in the provided bag and then into a prepaid FedEx package that will be delivered right to a GENETWORx lab.

Receive Results in Less Than 72 Hours
If the patient signed a release you will receive the results via the GENETWORx portal in less than 72 hours. Patients can also access their results directly from GENETWORx via phone, text or the patient portal. GENETWORx will report to the State Health Department, so you do not have to report anything to local health authorities.
GENETWORx also offers COVID-19 Nasal Swab Test Kits which may be self-administered and sent to your employees’ homes or locations of choice. Click to learn more about the self-collection COVID-19 Nasal Swab Test Kit option.
Enroll for the GENETWORx ‘Test Everyone USA’ Program
Businesses need to test 100% of their employees for COVID-19 in order to:
- Re-open business
- Restore a productive workforce and welcome employees back to work
- Meet customer demand for goods/services and generate revenue
- Ensure customer and employee confidence by creating a safe and healthy environment
- Provide the data to local, state and federal government, as well as medical experts to aid in research
- Stimulate the local and national economy
- Stop the spread of the COVID-19 virus
The ‘Test Everyone USA’ program, offered exclusively by GENETWORx, provides employers and organizations ongoing testing. Through this program, every employee will be tested for COVID-19 every 10 days, and receive test results in less than 72 hours. With this knowledge, everyone can have peace of mind and take the necessary actions based on their test results. Employers can focus on running their business with a productive, confident workforce and serve their customers and communities with the ‘COVID-19 Free, GENETWORx Certified’ seal.
COVID-19 Diagnostic Testing FAQs
Physicians, hospitals, and any other United States healthcare provider can order GENETWORx’s 2019 Novel Coronavirus (COVID-19) test. Our tests are available for any patients who meet current guidance for evaluation of COVID-19 infection. Our nasopharyngeal tests feature 99% specificity and sensitivity to detect the presence of SAR-CoV-2, the virus responsible for COVID-19.
GENETWORx Laboratory provides both a nasopharyngeal test and a saliva test which can be conducted on-site. Both of these tests are extremely accurate: the nasopharyngeal test has a 99% sensitivity and specificity, and the saliva test yields an accuracy greater than 98%. GENETWORx also provides a self-collected Nasal Swab Test Kit which can be sent to the home of your employees or patients. Click to learn more about the self-collection kit.
Call 610-726-1205 and a GENETWORx representative will set you up in GENETWORx’s portal and send the kits to your facility. Within 72 hours, your facility will be set up in our portal and your test kits will arrive.
After a physician contacts GENETWORx, we will send either the nasopharyngeal or saliva testing kits to your facility. The nasopharyngeal kit will include a swab and the universal transport media in a vial to ensure RNA and DNA survival. The saliva kit will include a specimen collection funnel, tube, and universal transport media and a cap for the tube. Once the swab is taken by a healthcare provider (for nasopharyngeal) or is self-administered (for saliva), the vial and test order with the physician’s signature are placed in an individual bag, which is then placed into a larger bag. This is sent via FEDEX to our laboratory, where it will be tested, and results will be sent through the GENETWORx portal in less than 72 hours.
GENETWORX’s nasopharyngeal swab collection is collected using a synthetic nasopharyngeal swab and placed in a viral transport medium to be accepted for testing. Only one sample type per patient will be accepted. Appropriately sized swabs with synthetic tips can be used with the exception of calcium alginate tips, swabs with preservatives, and swabs with wood shafts. Viral transport media that is acceptable for the collection of influenza specimens can be used to transport swabs for COVID-19 testing.
GENETWORX’s saliva specimen is gathered by the individual collecting their saliva into the funnel until the amount of liquid saliva reaches the fill-to line. Only one sample type per patient will be accepted.
Once GENETWORX’s receives the provided sample, it will be tested and the result – whether it’s positive or negative – will be sent confidentially via our portal within less than 72 hours. You will receive instructions on how to set up the portal upon initial receipt of the COVID-19 test. Patients can also access their results directly from GENETWORx via phone, text or the patient portal.
In order to get quick, accurate results to patients, the following samples cannot be accepted for nasopharyngeal testing:
- Any swabs with calcium alginate, cotton tips, or wooden shafts
- Samples that have been refrigerated for more than 72 hours
- Samples that have been kept at room temperature for more than 24 hours
- Samples that are improperly labeled
- Samples that are contaminated
- Broken or leaking transport devices
For saliva tests, please note:
- You CANNOT eat, drink, smoke, or chew gum 30 minutes before providing your sample
- Do not remove the plastic film from the funnel lid
Genetworx is uniquely qualified to perform COVID-19 testing and deliver incredibly accurate, timely results because we regularly perform complex molecular diagnostic testing, outside of the pandemic. Not all companies have the technology and infrastructure to deliver an accurate result in a timely manner. We have special instrumentation and expertise to run a large volume of COVID-19 tests and produce results in less than 72 hours. Our nasopharyngeal test yields over 99% specificity and sensitivity and our saliva test has a greater than 98% accuracy. This capability is not found in most tests or in most laboratories. Some tests without this level of sensitivity will not detect the presence of the virus.
Depending on your state, GENETWORx is required by law to report positive and/or negative results to the state and public health departments.
GENETWORx’s is currently performing thousands of COVID-19 tests every day. We have been working closely with local and state officials to continue providing our services during this pandemic. Every day, GENETWORx is adding machinery automation and efficiencies to continually increase our testing capacity to meet the demand during this pandemic.
COVID-19 Antibody Testing FAQs
GENETWORx COVID-19 Total Antibody (IgM & IgG) test is 99.8% specific and 100% sensitive when performed at least 14 days after COVID-19 infection.
Contact GENETWORx by calling 610-726-1205 to order your Total Antibody test. You’ll have a testing kit within 24 hours.
Yes. If you believe you’ve been exposed to COVID-19 or have tested positive but do not have symptoms, you can still get a GENETWORx Antibody test.
If you’ve tested positive for COVID-19, been at risk for exposure, have had symptoms or not, you are eligible for COVID-19 antibody testing.
There is no age minimum for antibody testing.
Although having the antibodies does not guarantee immunity, early research indicates there is at least some protection from previous COVID-19 exposure.
Because antibodies play such an important role in the battle against COVID-19, healthcare professionals need to know who has been exposed to COVID-19 and if they’ve developed an immune response. As we learn more and more about COVID-19, these results, combined with other important data, could help figure out individuals who are more (or less) susceptible to more COVID-19 infections.
If you test positive for COVID-19 antibodies, it likely means that you have been exposed to COVID-19. Antibodies are often the immune system’s way to fight off infections. However, antibodies don’t guarantee immunity against certain viruses. Unfortunately, we don’t know if people who have COVID-19 antibodies are protected in the future. Research is still ongoing.
Yes, you will want to social distance if you have COVID-19 antibodies. Just because you have antibodies doesn’t mean you’re immune from COVID-19 infection. Plus, you can transport the virus, increasing the risk of infection to other people.
No, you don’t have to get sick in order to get antibodies. Research shows that a large amount of people have the antibodies to COVID-19 without ever actually getting sick. That’s why some people test positive for COVID-19 without ever having any symptoms.
Simply having the presence of antibodies doesn’t mean you are immune against future COVID-19 infections. Antibody testing provides a yes or no when it comes to creation of antibodies to a specific virus.
Currently, there are no known medical risks associated with taking the COVID-10 Antibody test, beyond that of a typical blood draw.